What's New
Front Page
Aprinoia Therapeutics Receives Fast Track Designation for PET Tracer for PSP Diagnosis
Biopharmaceutical company Aprionoia Therapeutics announced that it has received an FDA Fast Track Designation for an imaging…
Dear HelpLine – Support for Young Adults
Dear HelpLine, My mom was diagnosed with FTD and I’m looking to connect with other young adults…
Advancing Hope: AFTD Joins National Institutes of Health AMP ALS Initiative as Partner
AFTD is joining fellow nonprofits, biopharmaceutical companies, and the U.S. Food and Drug Administration (FDA) as partners…
New York FTD Registry Bill Passes State Senate
A bill establishing a registry of FTD diagnoses in New York State unanimously passed the state Senate…
AFTD Board Member Kristin Holloway Tells Her FTD Story to Self Magazine
AFTD Board member Kristin Holloway shared her experiences as a care partner for her husband, Lee, in…
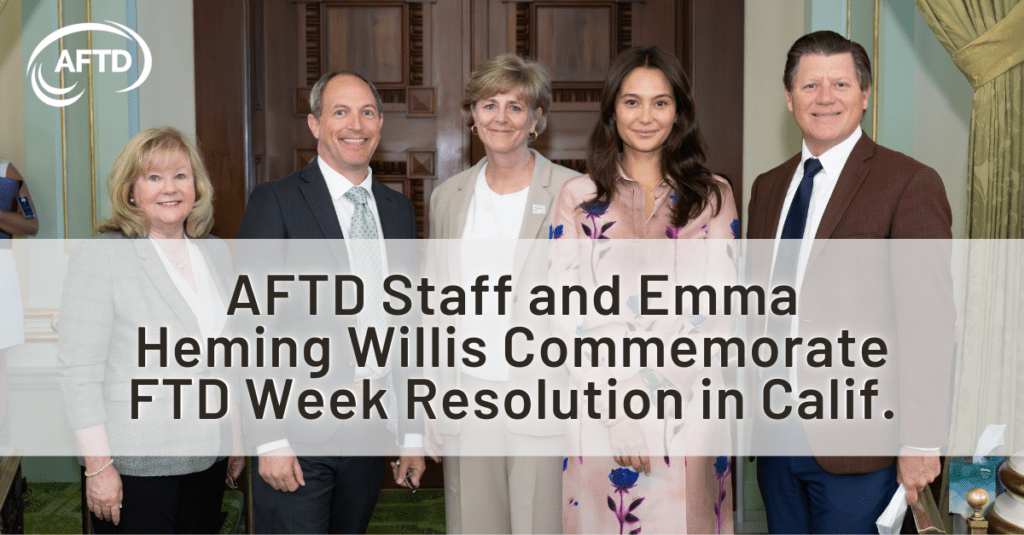
AFTD Staff and Emma Heming Willis Commemorate FTD Week Resolution in Calif.
(pictured above, L-R: FTD advocate Wanda Smith, Calif. Assemblymember Brian Maienschein, AFTD CEO Susan L-J Dickinson, FTD…
Session Videos Now Available from AFTD 2024 Education Conference
Videos from the AFTD 2024 Education Conference are now available on AFTD’s YouTube channel. Whether you were unable…
Passage Bio Highlights Promising Clinical Trial Progress in Quarterly Update
Biopharmaceutical company Passage Bio highlighted promising progress in its phase 1/2 clinical trial for a form of…
Advancing Hope: AFTD Attends 2024 Target ALS Annual Meeting
AFTD attended the 2024 Target ALS Meeting on May 7-9 in Boston, Massachusetts. There were over 800…
Help & Hope: The new FTD Disorders Registry Platform is Now Live!
The FTD Disorders Registry announced the launch of their updated platform at the AFTD 2024 Education Conference…